Implantable Cardioverter Defibrillators Approved for MRI Scanning
By MedImaging International staff writers
Posted on 23 Oct 2013
A new implantable cardioverter-defibrillator (ICD) is designed to increase the success rate of the first shock and reduce the need for subsequent shocks, and is the only device approved for magnetic resonance imaging (MRI) that is also able to offer 45 joules. The system therefore optimizes safety for both physicians and patients, offering them the promise of successful therapy from the very beginning. Posted on 23 Oct 2013
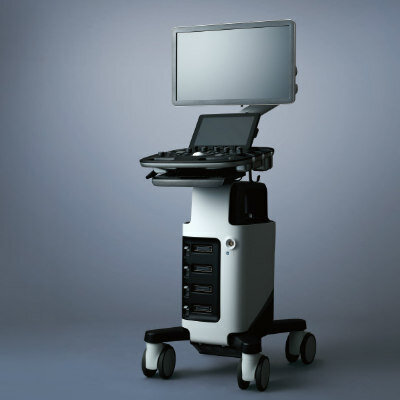
The first worldwide implantations of the ultrahigh-energy device taking place in Italy and Austria. Biotronik (Berlin, Germany), a manufacturer of ICDs and cardiac resynchronization therapy defibrillators (CRT-Ds).The new technology offers ultrahigh-energy therapy without compromising on short charge times (10 sec), smaller size (34 cc), and an excellent long life of more than 11 years. Designed to stop life-threatening arrhythmias with the very first shock, Idova 7 gives patients peace of mind.

Image: The Idova 7 ICD, designed to stop life-threatening arrhythmias with the very first shock (Photo courtesy of Biotronik).
“Ideally, a patient’s heart would react the same way to ICD therapy in real life as it would when tested after implantation. But that is not so often the case,” commented Dr. Clemens Steinwender, General Hospital Linz (Austria). “If I can give my patients that extra energy at the first shock, then I can assure them they are on the safe side.”
For ICD therapy to be effective, it is crucial to be aware of a patient’s defibrillation threshold (DFT), defined as the amount of energy required to shock the heart back to a normal rhythm. But DFT has a tendency to rise over time, and require higher amounts of energy. Higher DFTs can be due to factors as severely dilated hearts, widespread as prescribed medication, or young age and high activity levels.
“There are many variables that are related to the patient’s health and lifestyle, and not under my direct control. These can unfortunately affect defibrillation thresholds without our advanced knowledge,” explained Dr. Fabrizio Caravati, Hospital Fondazione Macchi (Varese, Italy). “It is important to be able to reassure patients that they have the maximum amount of energy at their disposal, in the event they should need it,” continued Dr. Paolo Bonfanti, Hospital Fondazione Macchi of Varese, Italy.
“After launching Ilesto and Iforia, we can now broaden our second generation of ProMRI devices with the ultrahigh-energy Idova 7 series,” commented Christoph Böhmer, president international at Biotronik. “Like all innovative Biotronik implants, Idova 7 will also be available with Biotronik Home Monitoring, which rapidly detects clinically relevant events like arrhythmias and can thereby prevent stroke and reduce hospitalization. In fact, the results of the recent IN-TIME study, introduced at this year’s ESC conference, demonstrated a significant reduction in all-cause mortality in heart failure patients with implant-based remote monitoring.”
Biotronik has introduced several innovations onto the market--including remote monitoring with Biotronik Home Monitoring in 2000 and the world’s first implantable cardioverter-defibrillators and implantable heart failure therapy devices with ProMRI technology.
The technology approved for MR scanning in 2012.
Related Links:
Biotronik