Unique Innovative in-Procedure Ablation Confirmation Software Receives FDA Clearance
|
By MedImaging International staff writers Posted on 08 Sep 2015 |
The first and only in-procedure ablation confirmation software that is part of an ablation system, and is intended for the ablation of soft tissue lesions, has received clearance from the US Food and Drug Administration (FDA; Silver Spring, MD USA).
The software was developed by a privately held medical device company and is intended for use with their ablation system. The software uses images from Computed Tomography (CT) scanners and Picture Archiving and Communication Systems (PACS) and helps clinicians identify ablation targets, assess correct placement of ablation probes, and confirm post procedure ablation zones.
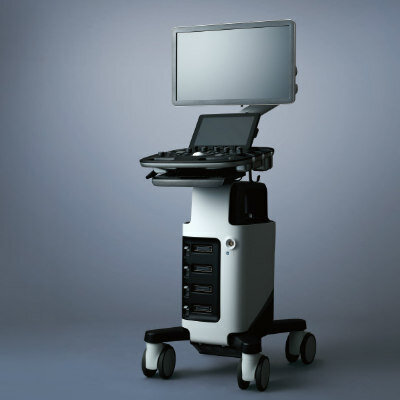
The Ablation Confirmation (AC) software, user interface, and NewWave Intelligent Ablation system were developed by NewWave Medical (Madison WI, USA). The AC software imports images from a CT scanner and PACS, and displays them on a dedicated monitor on the NewWave Intelligent Ablation system.
Dan Sullivan, CEO NeuWave Medical, said, “This is a significant step forward, as today physicians performing an ablation have to view patient CT scans with the naked eye on side by side monitors outside the procedure room. Now, with Ablation Confirmation, only available from NeuWave, there is no need to imagine what the ablation scans look like. The system will overlay pre‐ and post‐ablation scans to show the physician whether or not the ablation is complete, all while never leaving the procedure room. This is game changing for physicians and expected to set a new standard of care for the ablation of soft tissue lesions.”
Related Links:
NewWave Medical
The software was developed by a privately held medical device company and is intended for use with their ablation system. The software uses images from Computed Tomography (CT) scanners and Picture Archiving and Communication Systems (PACS) and helps clinicians identify ablation targets, assess correct placement of ablation probes, and confirm post procedure ablation zones.
The Ablation Confirmation (AC) software, user interface, and NewWave Intelligent Ablation system were developed by NewWave Medical (Madison WI, USA). The AC software imports images from a CT scanner and PACS, and displays them on a dedicated monitor on the NewWave Intelligent Ablation system.
Dan Sullivan, CEO NeuWave Medical, said, “This is a significant step forward, as today physicians performing an ablation have to view patient CT scans with the naked eye on side by side monitors outside the procedure room. Now, with Ablation Confirmation, only available from NeuWave, there is no need to imagine what the ablation scans look like. The system will overlay pre‐ and post‐ablation scans to show the physician whether or not the ablation is complete, all while never leaving the procedure room. This is game changing for physicians and expected to set a new standard of care for the ablation of soft tissue lesions.”
Related Links:
NewWave Medical
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreMRI
view channel
PET/MRI Improves Diagnostic Accuracy for Prostate Cancer Patients
The Prostate Imaging Reporting and Data System (PI-RADS) is a five-point scale to assess potential prostate cancer in MR images. PI-RADS category 3 which offers an unclear suggestion of clinically significant... Read more
Next Generation MR-Guided Focused Ultrasound Ushers In Future of Incisionless Neurosurgery
Essential tremor, often called familial, idiopathic, or benign tremor, leads to uncontrollable shaking that significantly affects a person’s life. When traditional medications do not alleviate symptoms,... Read more
Two-Part MRI Scan Detects Prostate Cancer More Quickly without Compromising Diagnostic Quality
Prostate cancer ranks as the most prevalent cancer among men. Over the last decade, the introduction of MRI scans has significantly transformed the diagnosis process, marking the most substantial advancement... Read moreUltrasound
view channel
Deep Learning Advances Super-Resolution Ultrasound Imaging
Ultrasound localization microscopy (ULM) is an advanced imaging technique that offers high-resolution visualization of microvascular structures. It employs microbubbles, FDA-approved contrast agents, injected... Read more
Novel Ultrasound-Launched Targeted Nanoparticle Eliminates Biofilm and Bacterial Infection
Biofilms, formed by bacteria aggregating into dense communities for protection against harsh environmental conditions, are a significant contributor to various infectious diseases. Biofilms frequently... Read moreNuclear Medicine
view channel
New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
The development of lead-212 (212Pb)-PSMA–based targeted alpha therapy (TAT) is garnering significant interest in treating patients with metastatic castration-resistant prostate cancer. The imaging of 212Pb,... Read moreNew Radiotheranostic System Detects and Treats Ovarian Cancer Noninvasively
Ovarian cancer is the most lethal gynecological cancer, with less than a 30% five-year survival rate for those diagnosed in late stages. Despite surgery and platinum-based chemotherapy being the standard... Read more
AI System Automatically and Reliably Detects Cardiac Amyloidosis Using Scintigraphy Imaging
Cardiac amyloidosis, a condition characterized by the buildup of abnormal protein deposits (amyloids) in the heart muscle, severely affects heart function and can lead to heart failure or death without... Read moreGeneral/Advanced Imaging
view channel
New AI Method Captures Uncertainty in Medical Images
In the field of biomedicine, segmentation is the process of annotating pixels from an important structure in medical images, such as organs or cells. Artificial Intelligence (AI) models are utilized to... Read more.jpg)
CT Coronary Angiography Reduces Need for Invasive Tests to Diagnose Coronary Artery Disease
Coronary artery disease (CAD), one of the leading causes of death worldwide, involves the narrowing of coronary arteries due to atherosclerosis, resulting in insufficient blood flow to the heart muscle.... Read more
Novel Blood Test Could Reduce Need for PET Imaging of Patients with Alzheimer’s
Alzheimer's disease (AD), a condition marked by cognitive decline and the presence of beta-amyloid (Aβ) plaques and neurofibrillary tangles in the brain, poses diagnostic challenges. Amyloid positron emission... Read more.jpg)
CT-Based Deep Learning Algorithm Accurately Differentiates Benign From Malignant Vertebral Fractures
The rise in the aging population is expected to result in a corresponding increase in the prevalence of vertebral fractures which can cause back pain or neurologic compromise, leading to impaired function... Read moreIndustry News
view channel
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more